|
1. General fullerenes, graphs, symmetry and isomers From the tetravalence of C result four possible vertex situations,
that could be denoted as 31, 22, 211 and 1111 (Fig.
1a). The situation 31 could be obtained by adding two C atoms between
any two others connected by a single bond, and situation 22 by adding a
C atom between any two others connected by a double bond (Fig.
1b). Therefore, we could restrict our consideration to the remaining
two non-trivial cases: 211 and 1111. Working in opposite sense, we could
always delete 31 or 22 vertices, and obtain a reduced 4-regular graph,
where in each vertex occurs at most one double bond (digon), that could
be denoted by colored (bold) edge (Fig.
1a). First, we could consider all 4-regular graphs on a sphere, from
which non-trivial in the sense of derivation are only reduced ones. In
the knot theory, 4-regular graphs on a sphere with all vertices of the
type 1111 are known as "basic polyhedra" [1,2,
3,4],
and that with at least one vertex with a digon as "generating knots or
links" [4]. From the chemical reasons,
the vertices of the type 1111 are only theoretically acceptable. If all
the vertices of such 4-regular graph are of the type 211, such graph we
will be called a general fullerene. Every general fullerene could
be derived from a basic polyhedron by "vertex bifurcation", this means,
by replacing its vertices by digons, where for their position we have always
two possibilities (Fig. 1c). To
every general fullerene corresponds (up to isomorphism) an edge-colored
3-regular graph (with bold edges denoting digons). This way, we have two
complementary ways for the derivation of general fullerenes: vertex bifurcation
method applied to basic polyhedra, or edge-coloring method applied to 3-regular
graphs, where in each vertex there is exactly one colored edge. For every
general fullerene we could define its geometrical structure (i.e. the positions
of C atoms) described by a non-colored 3-regular graph, and its chemical
structure (i.e. positions of C atoms and their double bonds) described
by the corresponding edge-colored 3-regular graph. In the same sense, for
every general fullerene we could distinguish two possible symmetry groups:
a symmetry group G corresponding to the geometrical structure
and its subgroup G' corresponding to the chemical structure.
In the same sense, we will distinguish geometrical and chemical
isomers. For example, for C60, G=G'=[3,5]=Ih
=S5 of order 120 [5],
but for C80 with the same G, G' is always a proper
subgroup of G, and its chemical symmetry is lower than the geometrical.
Hence, after C60, the first fullerene with G=G'=
[3,5]=Ih=S5
will be C180, then C240, etc.
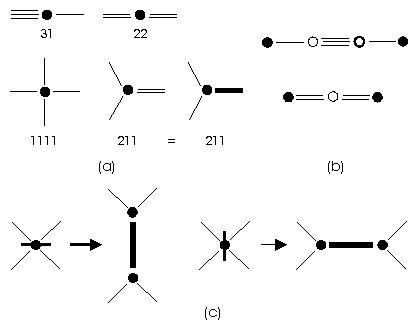 Working with general fullerenes without any restriction
for the number of edges of their faces, the first basic polyhedron from
which we could derive them (after the trivial 1*) will be the regular octahedron
{3,4} or 6*, from which we obtain 7 general fullerenes. From
the basic polyhedron 8* with v=8 we derive 30, and from
the basic polyhedron 9* we obtain 4 general fullerenes. All
the basic polyhedra with v<13 and their
Schlegel diagrams are given by Fig. 2.
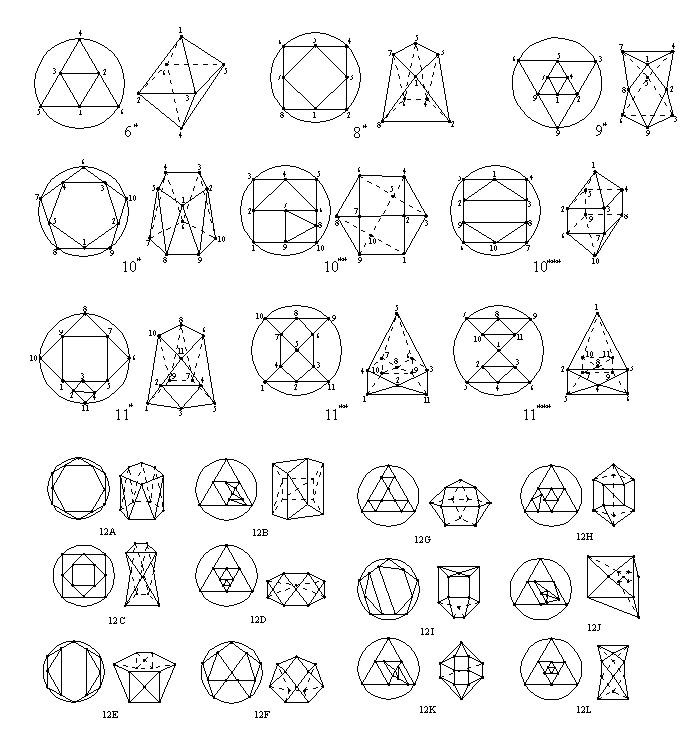 |